How many milliliters of 12.0 M HCl(aq) must be diluted with water to make exactly 500. mL of 3.00 M hydrochloric acid? | Socratic

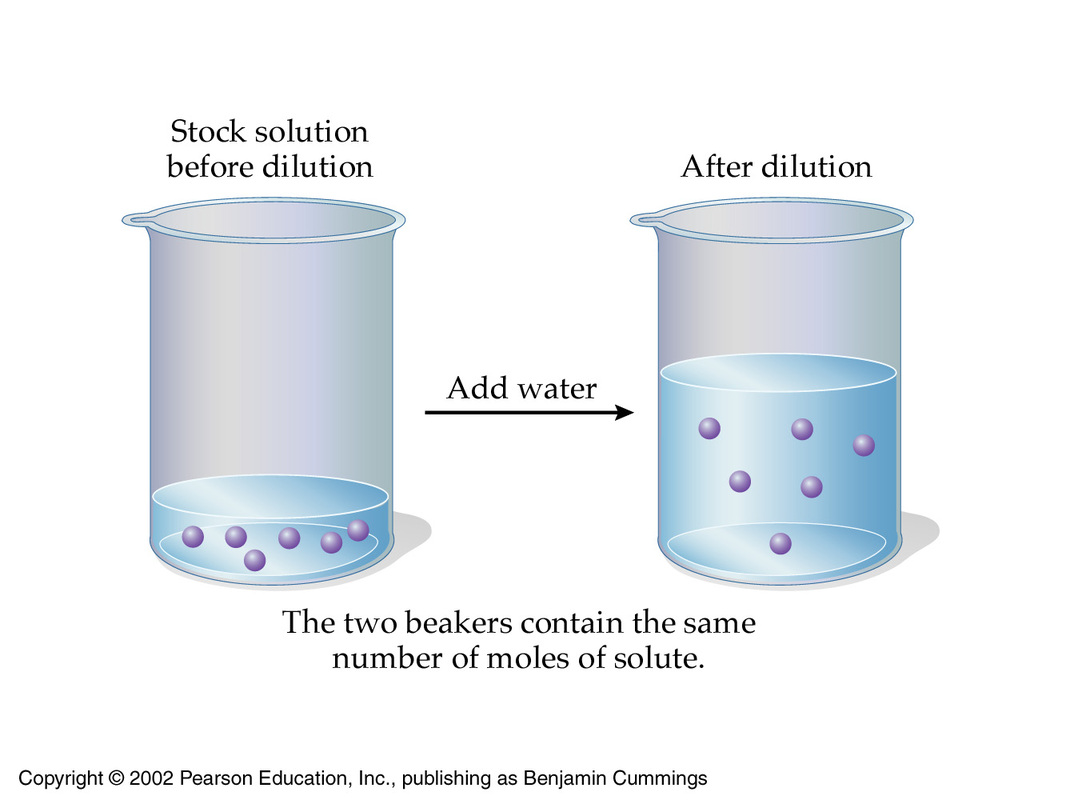
SOLVED: I would dilute 100.0 mL of a 5.0 M HCl solution to make a 0.20 M HCl solution. What will be the final volume? (2 pts) 10. Calculate the molarity of

How to Prepare 1M HCl Solution | Preparation of 0.1M HCl Solution | Hydrochloric acid 0.1 M Solution - YouTube
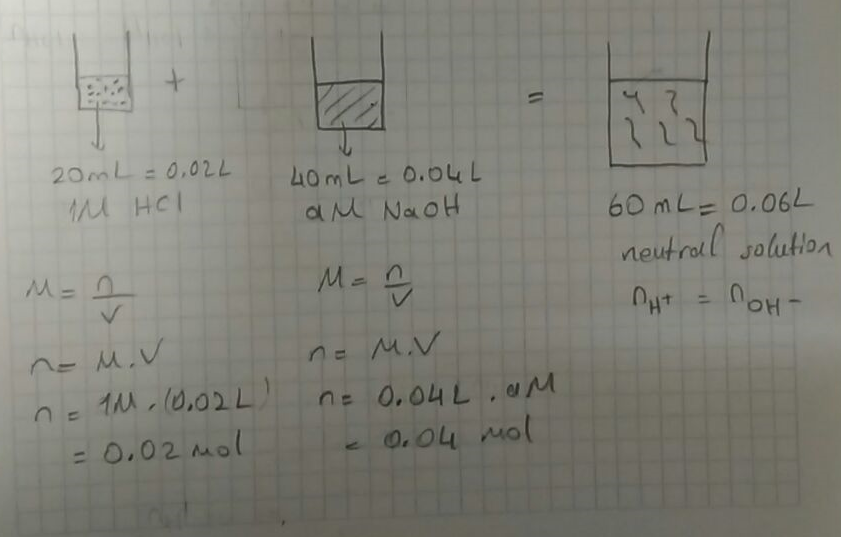
If 20 mL of 1.0 M HCl is used completely to neutralize 40 mL of an NaOH solution, what is the molarity of the NaOH solution? | Socratic
1 M NH4OH and 1 M HCl are mixed to make total volume of 300 mL. If pH of the mixture is 9.26 and pKa (NH4^+) = 9.26 - Sarthaks eConnect | Largest Online Education Community

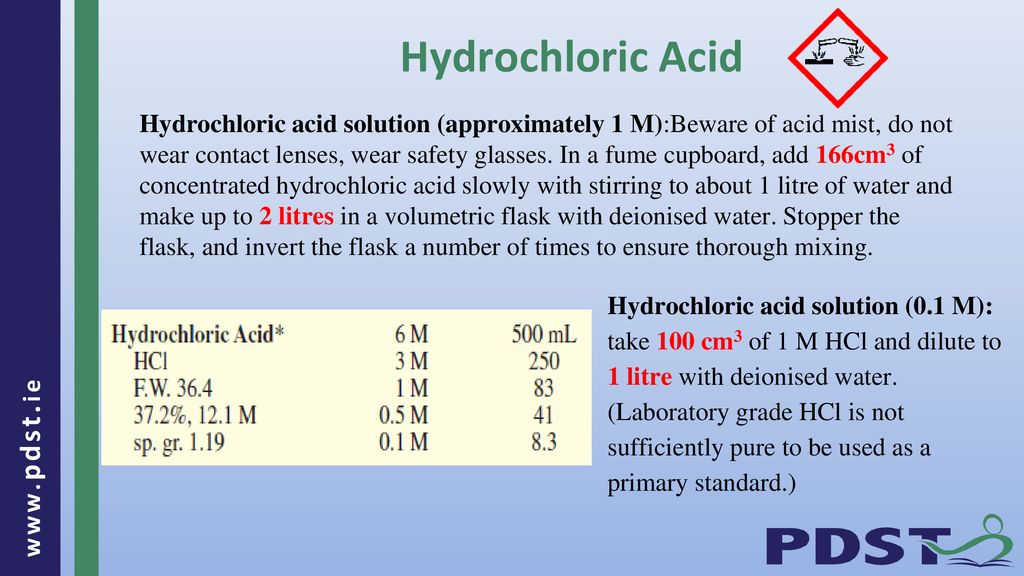
Discussion | 31 5. How many grams are needed to make 2.5L of 1.5M HCl solution? CI 350 レ. 6. How many grams are needed to make 500...

How to Prepare 1 molar HCl from 37% of HCl having density 1.18 g/cm3. | Umair Khan Academy - YouTube

What volume of 12.5 M concentrated HCl is required to make 1 L of 0.1 M HCl solution?A. 7 mLB. 9 mLC. 8 mLD. 10 mL
If 250ml of 1M HCl is diluted to 1000ml, what would be molarity of the diluted solution? What will be the pH? - Quora

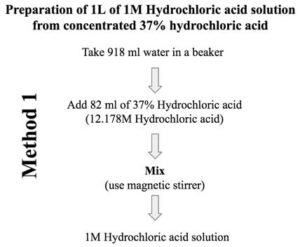
SOLVED: Solution Preparation and Standardization Name: Hydriodic Acid Calculations Calculate the volume of concentrated (12 M) HCI required to make 100.0 mL of a 1M solution: CcVr = CaVd (12 M)(Vr) = (